Diagnosis
Stage 4
Metastatic treatment
It is to be noted that although metastatic breast cancer is treatable and the goal of treatment is to help control cancer growth, it is usually not curable.1,3
Patients with HER2+ metastatic or locally recurrent unresectable breast cancer that has spread to other parts of the body may be given SIGRIMA™ along with Trastuzumab and docetaxel as initial treatment.1,2
This will be given to patients who have not received any anti-HER2 therapy or chemotherapy for metastatic breast cancer.2



It is to be noted that although metastatic breast cancer is treatable and the goal of treatment is to help control cancer growth, it is usually not curable.1,3
 Who can receive SIGRIMA™?
Who can receive SIGRIMA™?
Patients with HER2+ metastatic or locally recurrent unresectable breast cancer can be given SIGRIMA™ and Trastuzumab-based therapy if:
 How SIGRIMA™ + Trastuzumab-based therapy may help?
How SIGRIMA™ + Trastuzumab-based therapy may help?
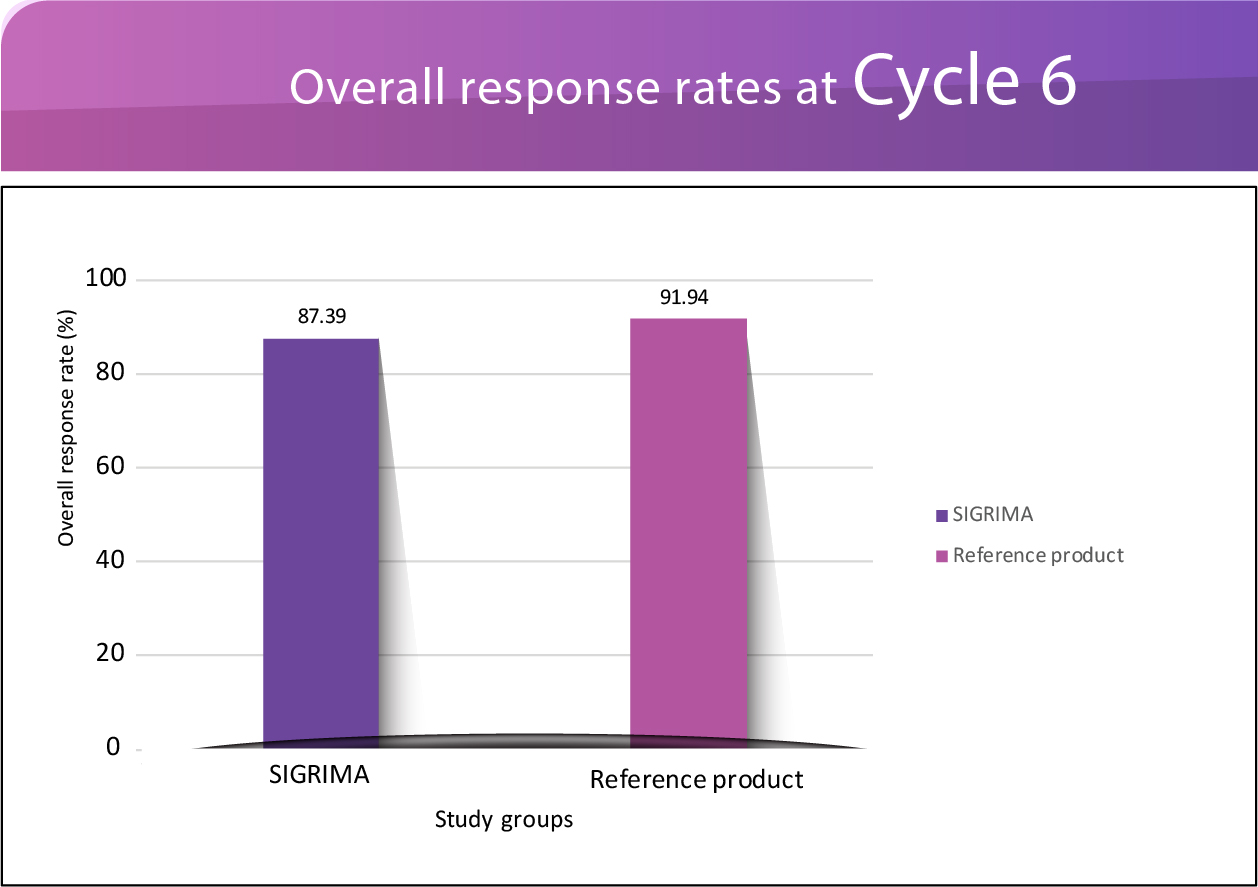
The study enrolled 268 patients with metastatic breast cancer. The results demonstrated that patients who received Test Pertuzumab (SIGRIMA™) experienced comparable efficacy, safety,
pharmacokinetics and immunogenicity to those given Reference Pertuzumab (Perjeta®, a product of Genentech, Inc.) over a duration of 155 days (approximately 22 weeks). This duration
included a 4-week screening period and a 106-day treatment period with 6 cycles of 21 days each.
In patients with metastatic breast cancer, post 6 cycles of
treatment, the study found that SIGRIMA™
was just as effective as the reference product Perjeta.5
TEAE, treatment-emergent adverse event.
If a subject has multiple occurrences of an AE or TEAE, the subject is presented only once for the corresponding AE or TEAE.6
N = number of subjects in specified treatment; n = number of subjects having non-missing values.6
The percentage calculated by (n/N)*100.6
Study Protocol
Efficacy Comparision
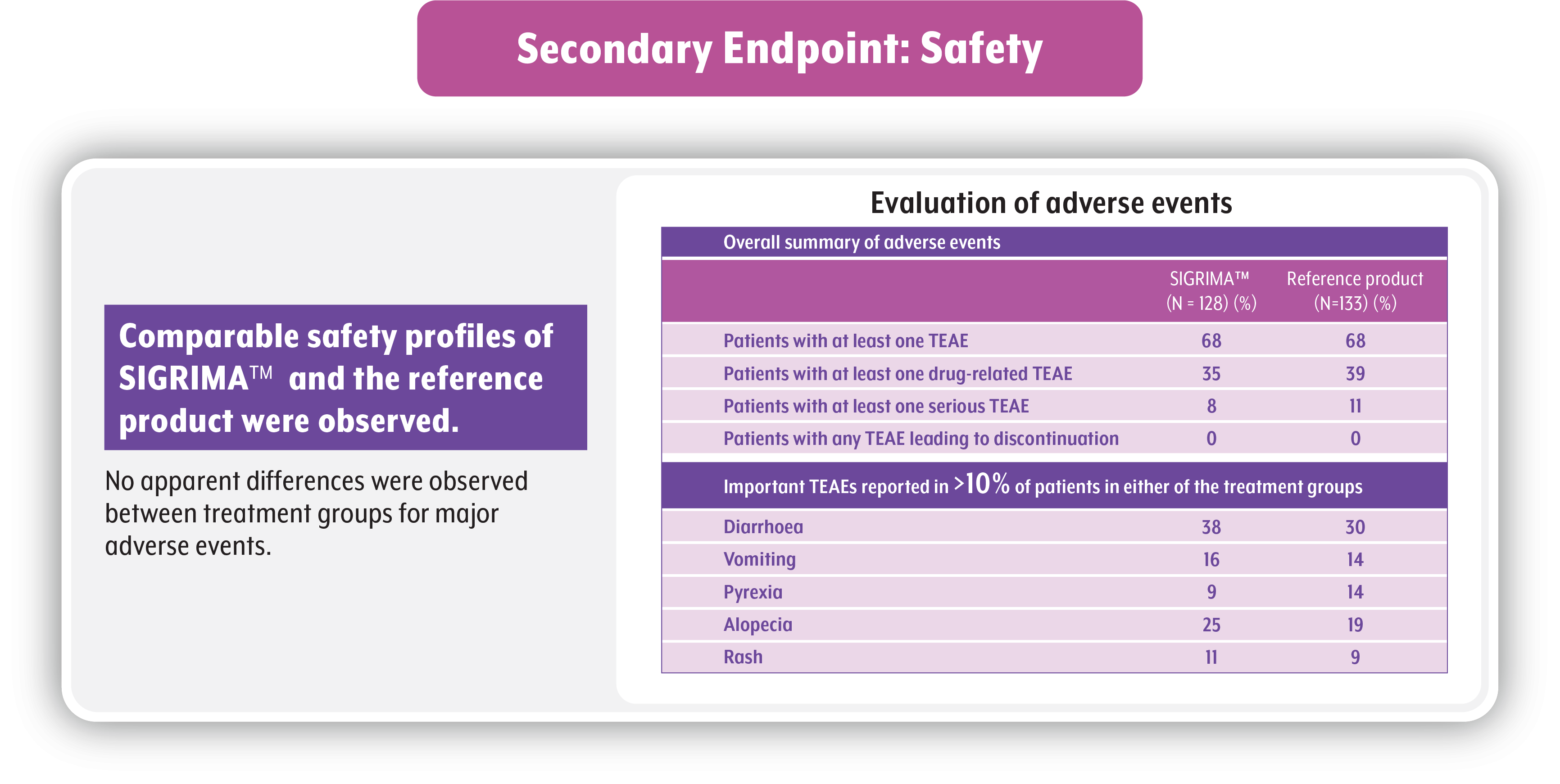
Safety Comparision6
 What are the most serious side effects of SIGRIMA™?
What are the most serious side effects of SIGRIMA™?
 What are the most serious side effects of SIGRIMA™?
What are the most serious side effects of SIGRIMA™? Are there any side effects of SIGRIMA™ I should look out for?
Are there any side effects of SIGRIMA™ I should look out for?
While the severe side effects of SIGRIMA™ are not experienced by everyone, side effects from SIGRIMA™ are common.1 So, it is important for you to know the symptoms and side effects one should look out for.
In case the side effects start becoming more serious, your doctor may stop the treatment. This is why you shouldimportantsafety report the side effects you are worried about to your doctor, and be sure to ask all your queries regarding the treatment.
 What are the most serious side effects of SIGRIMA™?
What are the most serious side effects of SIGRIMA™?SIGRIMA™, in some cases, may lead to cardiovascular (heart) problems,which may or may not cause symptoms. For example, congestive heart failure and decreased function of the heart.2,3
Your doctor may test your heart health before treating with SIGRIMA™ and will also monitor your heart function throughout the treatment. Based on the test results, your doctor may stop or pause the treatment with SIGRIMA™.
If you experience any of the following symptoms with SIGRIMA™, seek professional help immediately:
difficulty breathing (new onset or worsening of existing condition), face/ankle/leg swelling, heart palpitations, feeling dizzy, sudden excess weight gain (>2.2 kg) in 1 day, or loss of consciousness.4
 Can pregnant women take SIGRIMA™?
Can pregnant women take SIGRIMA™?
It is recommended to use birth control during your treatment with SIGRIMA™, which should be continued for 7 months after your last SIGRIMA™ dose.4
For breastfeeding women, it is important to talk to a doctor regarding the discontinuation of breastfeeding while on SIGRIMA™ or pausing treatment with SIGRIMA™.5
During your course of treatment with SIGRIMA™, if you are worried you might be pregnant, you should seek professional advice immediately.
 Are there any other serious side effects to look out for while taking SIGRIMA™?
Are there any other serious side effects to look out for while taking SIGRIMA™?You should not use SIGRIMA™ if you’re allergic to Pertuzumab or other ingredients found in SIGRIMA™. SIGRIMA™ is delivered via a needle directly into your vein4, which is why it can be linked to infusion-related reactions that may or may not be fatal in nature.7 Commonly observed reactions when receiving SIGRIMA™ + Trastuzumab + docetaxel include 4,8:
Commonly observed reactions when receiving ONLY SIGRIMA™ include,4:
Some people may also experience hypersensitivity reactions or anaphylaxis with SIGRIMA™, which are serious allergic reactions that can sometimes occur suddenly and are capable of affecting different areas of the body. These reactions have been reported to be fatal in some cases.9
 What are the commonly seen side effects of SIGRIMA™?
What are the commonly seen side effects of SIGRIMA™?The most commonly seen side effects of SIGRIMA™ + Trastuzumab + chemotherapy in those who are being treated for early breast cancer before surgery are4,10:
NOTE: Side effects experienced changes during the chemotherapy regimen.
Commonly seen side effects of SIGRIMA™ when given in combination with Trastuzumab and chemotherapy as part of an early breast cancer regimen post surgery are4,11:
Commonly seen side effects of SIGRIMA™ when given in combination with Trastuzumab and docetaxel for treatment of metastatic breast cancer (that has spread to different parts of the body) are4,8,12:
It is advised that you report any side effects to Zydus pharmacovigilance cell (Contact center: 1800 419 1141 / Email: drugsafety@zyduslife.com). You should speak to your healthcare professional to learn more about the benefits and risks associated with the use of SIGRIMA™. It is also advised that you check out the entire prescribing information of the medicine for additional safety information and other possible side effects.
 What is SIGRIMA™ used for treating?
What is SIGRIMA™ used for treating?
SIGRIMA™ (Pertuzumab) is a medicine prescribed by healthcare professionals in combination with Trastuzumab and chemotherapy13 in the following cases:
SIGRIMA™ (Pertuzumab) is a medicine prescribed by healthcare professionals and has been approved for treatment of people who have HER2+ metastatic breast cancer (that has spread to other parts of the body)10 and also who have not received anti-HER2 therapy or chemotherapy for metastatic breast cancer, in combination with Trastuzumab and docetaxel.4