Before surgery treatment: Early-stage breast cancer
Some individuals diagnosed with early breast cancer may find it beneficial to begin treatment before undergoing surgery. This is referred to as neoadjuvant treatment.1
SIGRIMA™ is approved for use alongside Trastuzumab and chemotherapy in neoadjuvant therapy for people with HER2+, locally advanced, inflammatory, or early-stage breast cancer (either greater than 2 cm in diameter or node-positive). This combination therapy constitutes an integral part of a complete treatment plan for early breast cancer (EBC).2
A primary objective of neoadjuvant treatment for early breast cancer is to decrease or eradicate cancer cells before undergoing surgery.1,3
It's important to note that not all cancers react to neoadjuvant treatment,4 and individuals may encounter various side effects, ranging from common to severe, during this treatment.5
Initiating therapy with SIGRIMA™ prior to surgery
SIGRIMA™ should be given every three weeks for a total of 3-6 cycles (9-18 weeks), along with Trastuzumab.2
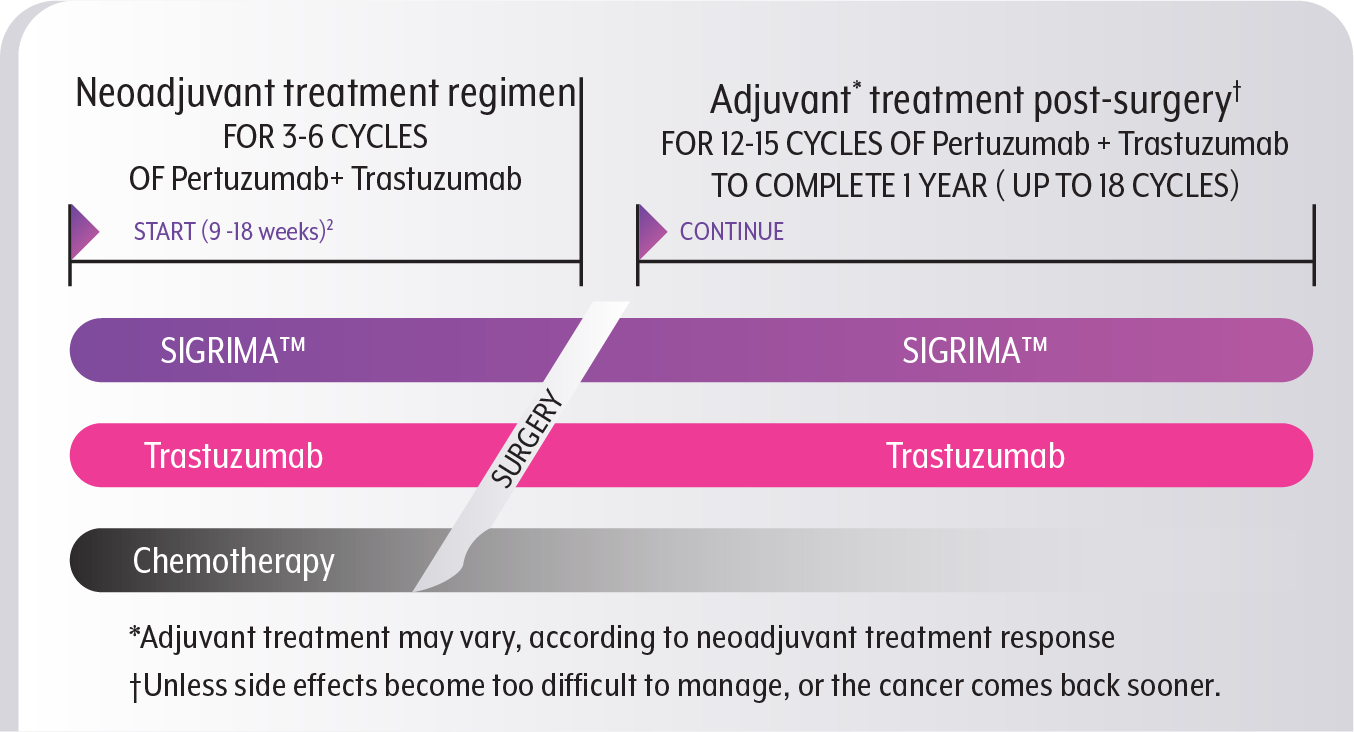
You will also undergo chemotherapy, though the specific dosing regimen and duration of cycles will be determined by the type of chemotherapy prescribed to you.6,7
A cycle is a regularly repeated treatment regimen with intervals of rest.7 For example, SIGRIMA™ + Trastuzumab is administered once every 3 weeks, constituting one cycle.
 What
What