 What are the commonly seen side effects of SIGRIMA™?
What are the commonly seen side effects of SIGRIMA™?
The most commonly seen side effects of SIGRIMA™ + Trastuzumab + chemotherapy in those who are being treated for early breast cancer before surgery are4,10:
- Constipation
- Diarrhea
- Decreased levels of RBCs
- Decreased levels of WBCs with/without fever
- Hair loss
- Headache
- Low platelet count
- Muscle pain
- Nausea and vomiting
- Nerve damage leading to symptoms like tingling feeling, numbness, or pain in limbs
- Rashes
- Weakness
NOTE: Side effects experienced changes during the chemotherapy regimen.
Commonly seen side effects of SIGRIMA™ when given in combination with Trastuzumab and chemotherapy as part of an early breast cancer regimen post surgery are4,11:
- Diarrhea
- Hair loss
- Nausea and vomiting
- Nerve damage leading to symptoms like tingling feeling, numbness, pain in limbs
- Tiredness
Commonly seen side effects of SIGRIMA™ when given in combination with Trastuzumab and docetaxel for treatment of metastatic breast cancer (that has spread to different parts of the body) are4,8,12:
- Decreased levels of WBCs with/without fever
- Diarrhea
- Hair loss
- Nausea
- Nerve damage leading to symptoms like tingling feeling, numbness, pain in limbs
- Tiredness
- Rash
It is advised that you report any side effects to Zydus pharmacovigilance cell (Contact center: 1800 419 1141 / Email: drugsafety@zyduslife.com).
You should speak to your healthcare professional to learn more about the benefits and risks associated with the use of SIGRIMA™. It is also advised that you check out the entire prescribing information of the medicine for additional safety information and other possible side effects.
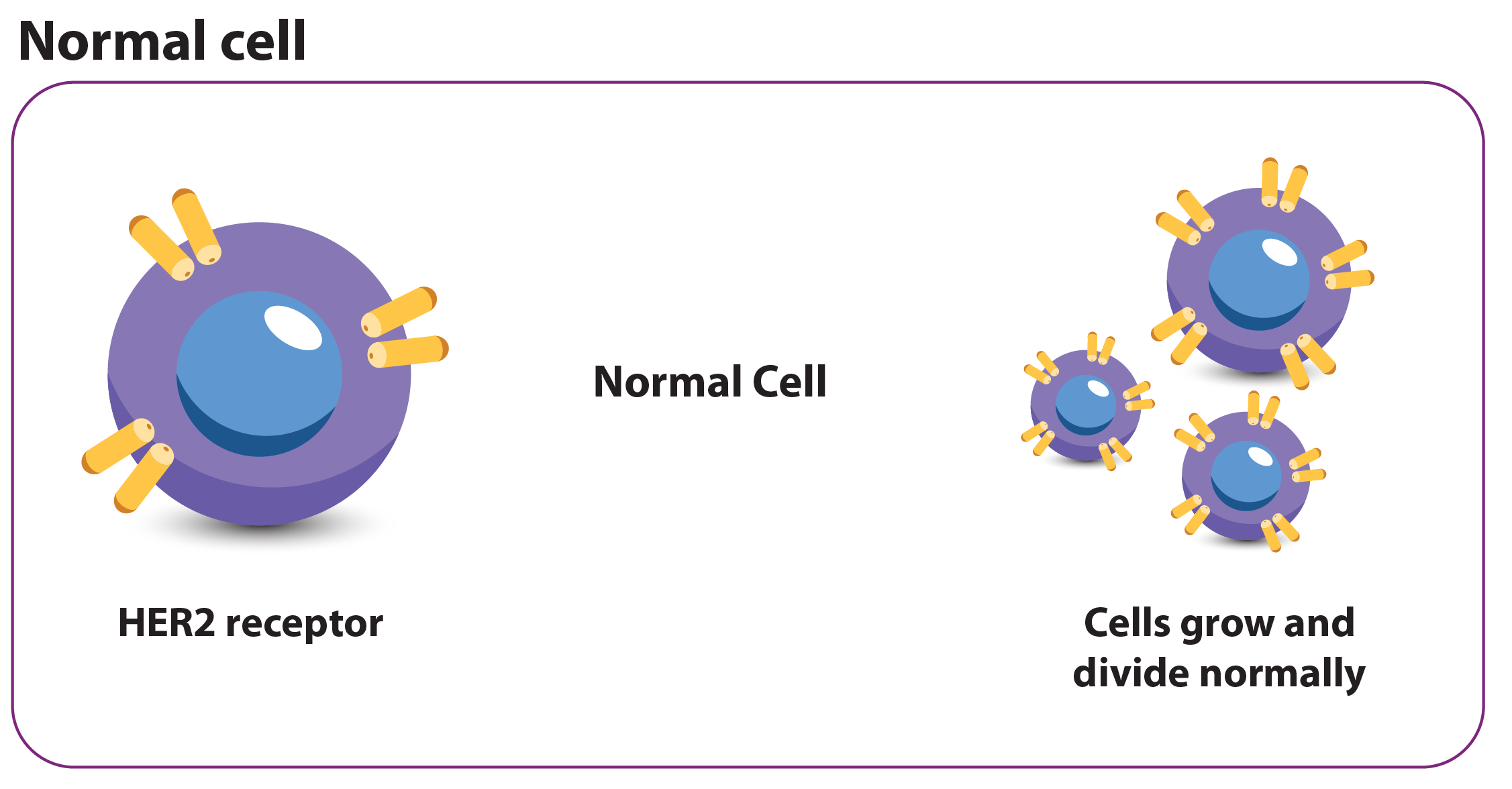
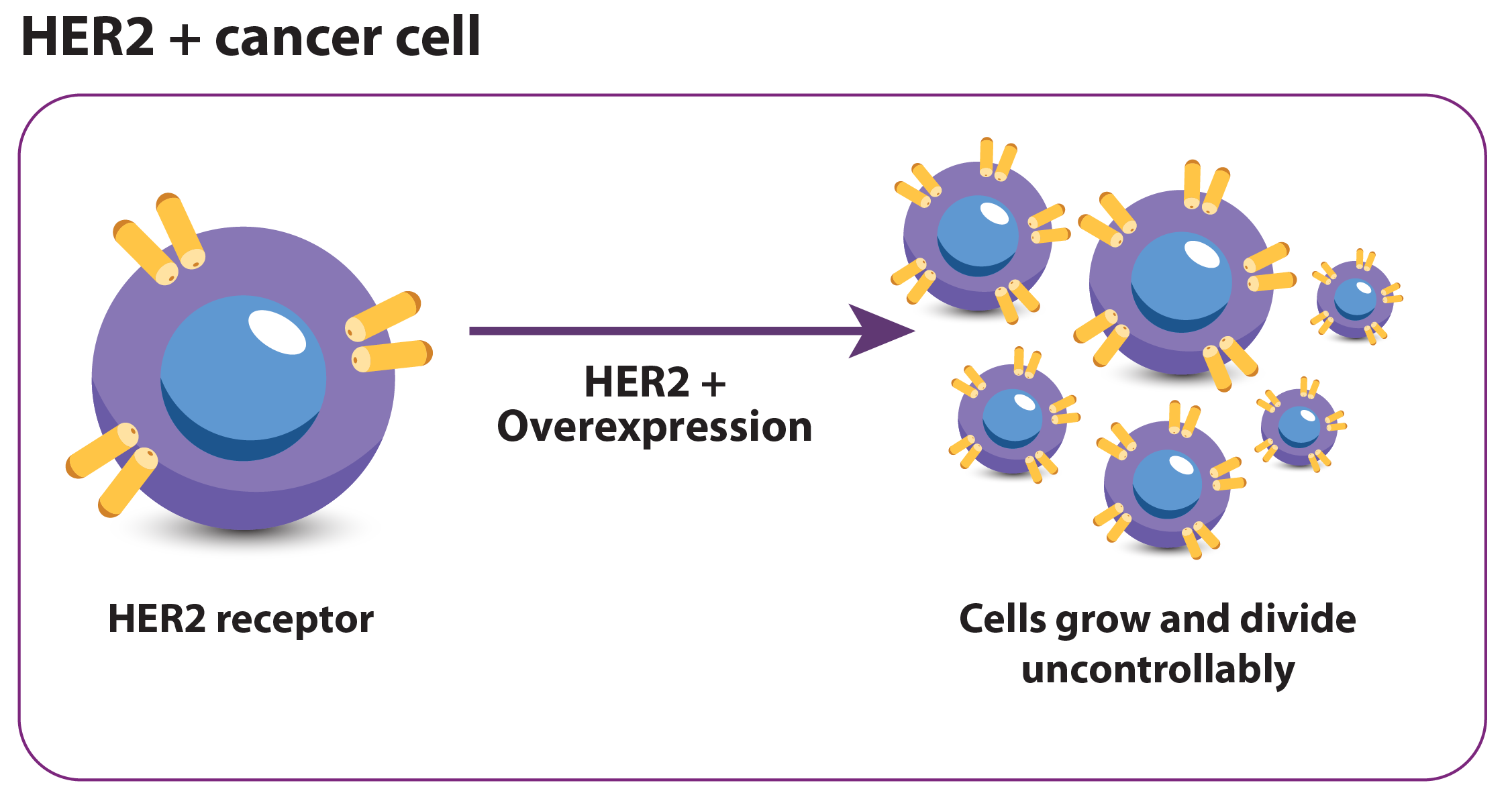
 What
What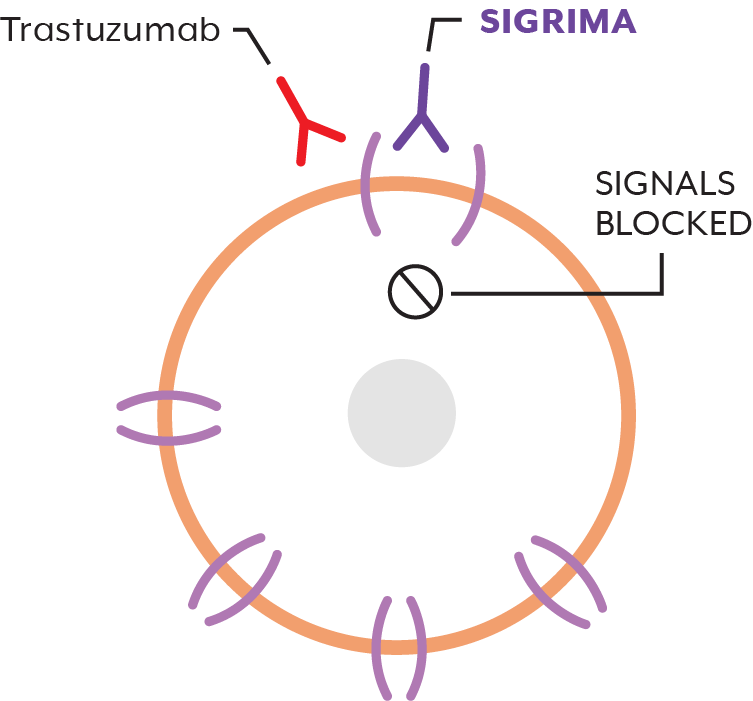
 Are
Are