After surgery treatment: Early-stage breast cancer
Adjuvant therapy is the treatment delivered to patients with early breast cancer after surgery.1
Following surgery for early breast cancer, SIGRIMA™ is approved for use in combination with Trastuzumab and chemotherapy for individuals with HER2+ early breast cancer at high risk of recurrence.2
Adjuvant treatment is administered to eliminate any remaining cancer cells in the body following surgery, thereby helping maintain a cancer free status for as long as possible.3,4
It is important to note that despite receiving adjuvant treatment, cancer recurrence is possible.5 In addition, individuals undergoing adjuvant treatment may experience a range of side effects, varying from common to more severe.6
SIGRIMA™-based Treatment after Surgery
If you have HER2+ early breast cancer, you might qualify for postsurgical treatment with a combination of SIGRIMA™ and Trastuzumab, provided that2
- You started SIGRIMA™ + Trastuzumab-based therapy before surgery
- Your cancer has a high risk of recurrence
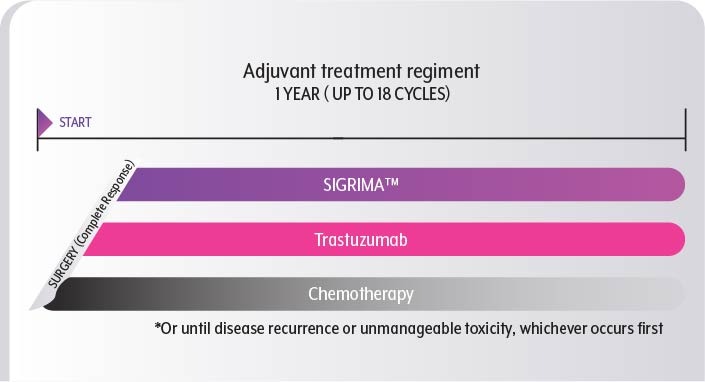
You are recommended to undergo a complete year of treatment with SIGRIMA™ and Trastuzumab, which amounts to up to 18 cycles. This duration includes any prior doses of SIGRIMA™ and Trastuzumab administered before surgery. However, your treatment may be halted earlier if the side effects become too challenging to handle or if the cancer returns.
In addition to SIGRIMA™ and Trastuzumab, you will receive chemotherapy, with the dosing schedule and number of cycles determined by the specific type of chemotherapy you are prescribed. Your healthcare provider will determine the most suitable chemotherapy regimen for your condition.7,8 a full year of SIGRIMA™ and Trastuzumab-based therapy is recommended, chemotherapy is often discontinued earlier.
A cycle refers to a treatment regimen that is repeated at regular intervals with breaks in between.7 For instance, SIGRIMA™ + Trastuzumab is administered once every 3 weeks, constituting one cycle.2
How many cycles do I have remaining?2
Use the information below to see how many SIGRIMA™ + Trastuzumab cycles you are left with:
SIGRIMA™ + Trastuzumab
CYCLES YOU HAVE RECEIVED
(before and/or after surgery)
*Unless the side effects become too challenging to handle or if the cancer recurs earlier than expected.1

How Pertuzumab + Trastuzumab-based therapy may help
Post-surgery treatment with Pertuzumab and Trastuzumab has demonstrated a reduction in the risk of cancer recurrence in specific individuals with HER2+ early breast cancer.9
Results of a clinical study with Pertuzumab10
In a clinical trial comprising 4804 patients with HER2+ early breast cancer, 2400 patients on adjuvant therapy with Pertuzumab + Trastuzumab showed an 23% lower risk of cancer recurrence compared to Trastuzumab-based treatment in 2404 patients. The trial included patients who started therapy post-surgery.9 Most of the participants were cancer free 8 years after the trial, regardless of the type of treatment they received. However, the patients who had lymph node positive disease, 86% of the participants treated with Pertuzumab + Trastuzumab were cancer-free compared to 81% cancer-free patients in the other group.1023% lowered risk of cancer recurrence9
Side Effects May Occur With SIGRIMA™
 What are the most serious side effects of SIGRIMA™?
What are the most serious side effects of SIGRIMA™?
- Treatment with SIGRIMA™ during pregnancy can harm the fetus, leading to death of the fetus, or may cause major birth defects.1
- SIGRIMA™ can cause heart problems, such as reduced heart function, which is asymptomatic, and congestive heart failure, which is symptomatic.2
 What are other possible serious side effects of SIGRIMA™?
What are other possible serious side effects of SIGRIMA™?
- SIGRIMA™ should be avoided in patients who are allergic to SIGRIMA™ or to any of the ingredients present in the SIGRIMA™ formulation.2
- Serious or fatal side effects may be related to infusion or allergic reactions (anaphylaxis/hypersensitivity).2
- The most common side effects of SIGRIMA™ in combination with Trastuzumab and docetaxel are low white blood cell count levels with or without fever, diarrhea, hair loss, excessive tiredness, nausea, rashes, and nerve damage causing weakness, numbness, and tingling in hands and feet.2
Important Safety Information
 Are there any side effects of SIGRIMA™ I should look out for?
Are there any side effects of SIGRIMA™ I should look out for?
While the severe side effects of SIGRIMA™ are not experienced by everyone, side effects from SIGRIMA™ are common.1 So, it is important for you to know the symptoms and side effects one should look out for.
In case the side effects start becoming more serious, your doctor may stop the treatment. This is why you shouldimportantsafety report the side effects you are worried about to your doctor, and be sure to ask all your queries regarding the treatment.
 What are the most serious side effects of SIGRIMA™?
What are the most serious side effects of SIGRIMA™?
SIGRIMA™, in some cases, may lead to cardiovascular (heart) problems,which may or may not cause symptoms. For example, congestive heart failure and decreased function of the heart.2,3
Your doctor may test your heart health before treating with SIGRIMA™ and will also monitor your heart function throughout the treatment. Based on the test results, your doctor may stop or pause the treatment with SIGRIMA™.
If you experience any of the following symptoms with SIGRIMA™, seek professional help immediately:
difficulty breathing (new onset or worsening of existing condition), face/ankle/leg swelling, heart palpitations, feeling dizzy, sudden excess weight gain (>2.2 kg) in 1 day, or loss of consciousness.4
 Can pregnant women take SIGRIMA™?
Can pregnant women take SIGRIMA™?
Treatment with SIGRIMA™ in pregnant women may lead to birth defects or the death of the unborn child.5,6
It is recommended to use birth control during your treatment with SIGRIMA™, which should be continued for 7 months after your last SIGRIMA™ dose.4
For breastfeeding women, it is important to talk to a doctor regarding the discontinuation of breastfeeding while on SIGRIMA™ or pausing treatment with SIGRIMA™.5
During your course of treatment with SIGRIMA™, if you are worried you might be pregnant, you should seek professional advice immediately.
 Are there any other serious side effects to look out for while taking SIGRIMA™?
Are there any other serious side effects to look out for while taking SIGRIMA™?
You should not use SIGRIMA™ if you’re allergic to Pertuzumab or other ingredients found in SIGRIMA™. SIGRIMA™ is delivered via a needle directly into your vein4, which is why it can be linked to infusion-related reactions that may or may not be fatal in nature.7 Commonly observed reactions when receiving SIGRIMA™ + Trastuzumab + docetaxel include 4,8:
- Tiredness
- Allergic reactions
- Muscle pain
- Vomiting
Commonly observed reactions when receiving ONLY SIGRIMA™ include,4:
- Fever and chills
- Headache
- Weakness
- Allergic reactions
- Vomiting
Some people may also experience hypersensitivity reactions or anaphylaxis with SIGRIMA™, which are serious allergic reactions that can sometimes occur suddenly and are capable of affecting different areas of the body. These reactions have been reported to be fatal in some cases.9
 What are the commonly seen side effects of SIGRIMA™?
What are the commonly seen side effects of SIGRIMA™?
The most commonly seen side effects of SIGRIMA™ + Trastuzumab + chemotherapy in those who are being treated for early breast cancer before surgery are4,10:
- Constipation
- Diarrhea
- Decreased levels of RBCs
- Decreased levels of WBCs with/without fever
- Hair loss
- Headache
- Low platelet count
- Muscle pain
- Nausea and vomiting
- Nerve damage leading to symptoms like tingling feeling, numbness, or pain in limbs
- Rashes
- Weakness
NOTE: Side effects experienced changes during the chemotherapy regimen.
Commonly seen side effects of SIGRIMA™ when given in combination with Trastuzumab and chemotherapy as part of an early breast cancer regimen post surgery are4,11:
- Diarrhea
- Hair loss
- Nausea and vomiting
- Nerve damage leading to symptoms like tingling feeling, numbness, pain in limbs
- Tiredness
Commonly seen side effects of SIGRIMA™ when given in combination with Trastuzumab and docetaxel for treatment of metastatic breast cancer (that has spread to different parts of the body) are4,8,12:
- Decreased levels of WBCs with/without fever
- Diarrhea
- Hair loss
- Nausea
- Nerve damage leading to symptoms like tingling feeling, numbness, pain in limbs
- Tiredness
- Rash
It is advised that you report any side effects to Zydus pharmacovigilance cell (Contact center: 1800 419 1141 / Email: drugsafety@zyduslife.com). You should speak to your healthcare professional to learn more about the benefits and risks associated with the use of SIGRIMA™. It is also advised that you check out the entire prescribing information of the medicine for additional safety information and other possible side effects.
 What is SIGRIMA™ used for treating?
What is SIGRIMA™ used for treating?
SIGRIMA™ (Pertuzumab) is a medicine prescribed by healthcare professionals in combination with Trastuzumab and chemotherapy13 in the following cases:
- For use before surgery as a neoadjuvant treatment14 in those with HER2+, locally advanced, inflammatory, or early-stage breast cancer (tumor size >2 cm in diameter or node-positive). For early breast cancer, SIGRIMA™ should be part of a complete therapy plan.4
- For use after surgery as an adjuvant treatment in those with HER2+ early breast cancer with a significant chance of returning back.4,15
SIGRIMA™ (Pertuzumab) is a medicine prescribed by healthcare professionals and has been approved for treatment of people who have HER2+ metastatic breast cancer (that has spread to other parts of the body)10 and also who have not received anti-HER2 therapy or chemotherapy for metastatic breast cancer, in combination with Trastuzumab and docetaxel.4